I. BENEFITS OF SOP
1. To provide people with all the safety, health, environmental and operational information necessary to perform a job properly. Placing value only on production while ignoring safety, health and environment is costly in the long run. It is better to train employees in all aspects of doing a job than to face accidents, fines and litigation later.
2. To ensure that production operations are performed consistently to maintain quality control of processes and products. Consumers, from individuals to companies, want products of consistent quality and specifications. SOPs specify job steps that help standardize products and therefore quality.
3. To ensure that processes continue uninterrupted and are completed on a prescribed schedule. By following SOPs, you help ensure against process shut-downs caused by equipment failure or other facility damage.
4. To ensure that no failures occur in manufacturing and other processes that would harm anyone in the surrounding community. Following health and environmental steps in SOPs ensures against spills and emissions that threaten plant neighbors and create community outrage.
5. To ensure that approved procedures are followed in compliance with company and government regulations. Well-written SOPs help ensure that government regulations are satisfied. They also demonstrate a company's good-faith intention to operate properly. Failure to write and use good SOPs only signals government regulators that your company is not serious about compliance.
6. To serve as a training document for teaching users about the process for which the SOP was written. Thorough SOPs can be used as the basis for providing standardized training for employees who are new to a particular job and for those who need re-training.
7. To serve as a checklist for co-workers who observe job performance to reinforce proper performance. The process of actively caring about fellow workers involves one worker coaching another in all aspects of proper job performance. When the proper procedures are outlined in a good SOP, any co- worker can coach another to help improve work skills.
8. To serve as a checklist for auditors. Auditing job performance is a process similar to observation mentioned in the previous item only it usually involves record keeping. SOPs should serve as a strong basis when detailed audit checklists are developed.
9. To serve as an historical record of the how, why and when of steps in an existing process so there is a factual basis for revising those steps when a process or equipment are changed. As people move from job to job within and between companies, unwritten knowledge and skills disappear from the workplace. Properly maintained written SOPs can chronicle the best knowledge that can serve new workers when older ones move on.
10. To serve as an explanation of steps in a process so they can be reviewed in accident investigations. Although accidents are unfortunate, view them as opportunities to learn how to improve conditions. A good SOP gives you a basis from which to being investigating accidents.
II. Ten reasons for writing SOPs:
1. To provide individuals who perform operations with all the safety, health, environmental and operational information required to perform a job properly
2. To protect the health and safety of employees, and to protect the environment
3. To protect the community
4. To ensure that operations are done consistently in order to maintain quality control of processes and products
5. To ensure that processes continue and are completed on a prescribed schedule
6. To ensure that no failures occur in manufacturing and related processes that would harm employees or anyone in the surrounding community
7. To ensure that approved procedures are followed in compliance with company and government regulations
8. To serve as a training document for teaching users about a process
9. To serve as an historical record of the how, why and when of steps in a process for use when modifications are made to that process and when a SOP must be revised
10. To serve as an explanation of steps in a process that can be reviewed in incident investigations that seek to improve safety practices and operating.
III. SOP PROCESS
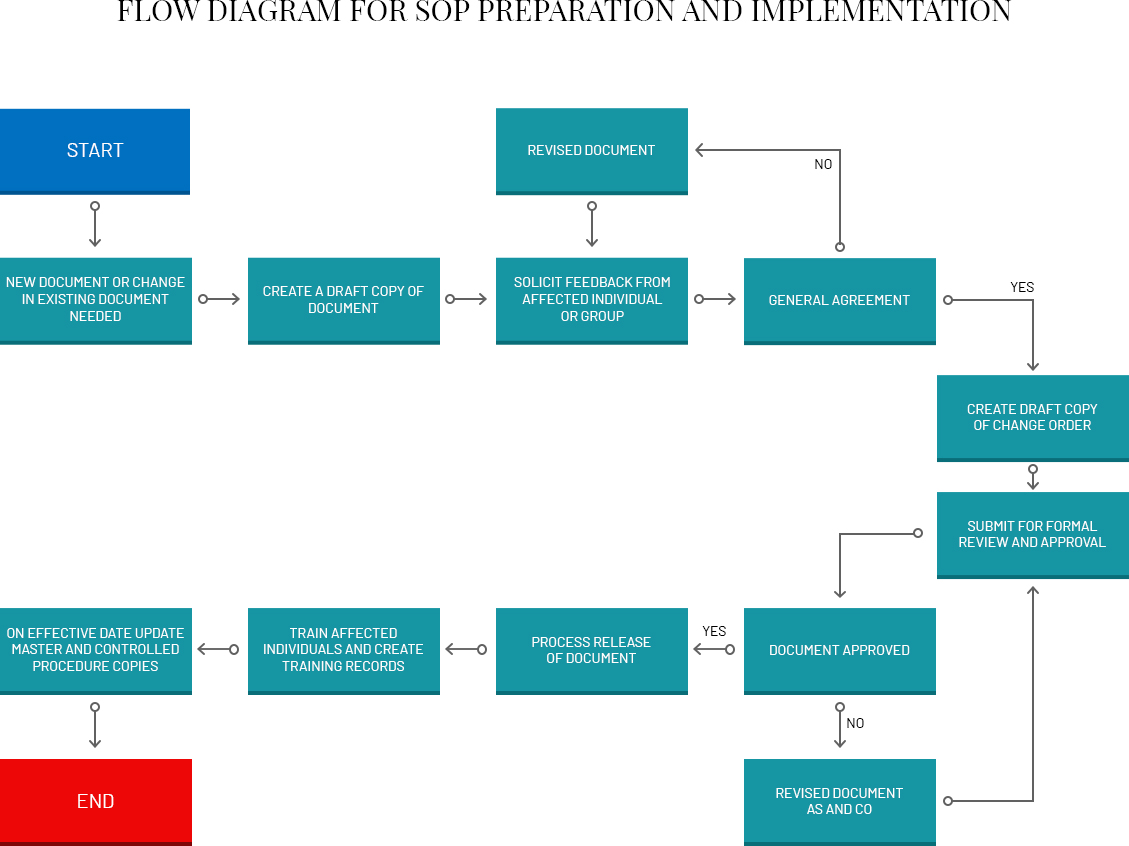
SOP Preparation
The organization should have a procedure in place for determining what procedures or processes need to be documented. Those SOPs should then be written by individuals knowledgeable with the activity and the organization's internal structure. These individuals are essentially subject-matter experts who actually perform the work or use the process. A team approach can be followed, especially for multi-tasked processes where the experiences of a number of individuals are critical, which also promotes “buy-in” from potential users of the SOP.
SOPs should be written with sufficient detail so that someone with limited experience with or knowledge of the procedure, but with a basic understanding, can successfully reproduce the procedure when unsupervised. The experience requirement for performing an activity should be noted in the section on personnel qualifications. For example, if a basic chemistry or biological course experience or additional training is required that requirement should be indicated.
SOP Review and Approval
SOPs should be reviewed (that is, validated) by one or more individuals with appropriate training and experience with the process. It is especially helpful if draft SOPs are actually tested by individuals other than the original writer before the SOPs are finalized.
The finalized SOPs should be approved as described in the organization‟s Quality Management Plan or its own SOP for preparation of SOPs. Generally the immediate supervisor, such as a section or branch chief, and the organization‟s quality assurance officer review and approve each SOP. Signature approval indicates that an SOP has been both reviewed and approved by management. As per the Government Paperwork Elimination Act of 1998, use of electronic signatures, as well as electronic maintenance and submission, is an acceptable substitution for paper, when practical.
Frequency of Revisions and Reviews
SOPs need to remain current to be useful. Therefore, whenever procedures are changed, SOPs should be updated and re-approved. If desired, modify only the pertinent section of an SOP and indicate the change date/revision number for that section in the Table of Contents and the document control notation.
SOPs should be also systematically reviewed on a periodic basis, e.g. every 1-2 years, to ensure that the policies and procedures remain current and appropriate, or to determine whether the SOPs are even needed. The review date should be added to each SOP that has been reviewed. If an SOP describes a process that is no longer followed, it should be withdrawn from the current file and archived.
Checklists
Many activities use checklists to ensure that steps are followed in order. Checklists are also used to document completed actions. Any checklists or forms included as part of an activity should be referenced at the points in the procedure where they are to be used and then attached to the SOP.
In some cases, detailed checklists are prepared specifically for a given activity. In those cases, the SOP should describe, at least generally, how the checklist is to be prepared, or on what it is to be based. Copies of specific checklists should be then maintained in the file with the activity results and/or with the SOP. Remember that the checklist is not the SOP, but a part of the SOP.
Document Control
Each organization should develop a numbering system to systematically identify and label their SOPs, and the document control should be described in its Quality Management Plan. Generally, each page of an SOP should have control documentation notation, similar to that illustrated below. A short title and identification (ID) number can serve as a reference designation. The revision number and date are very useful in identifying the SOP in use when reviewing historical data and is critical when the need for evidentiary records is involved and when the activity is being reviewed. When the number of pages is indicated, the user can quickly check if the SOP is complete. Generally this type of document control notation is located in the upper right-hand corner of each document page following the title page.
SOP Document Tracking and Archival
The organization should maintain a master list of all SOPs. This file or database should indicate the SOP number, version number, date of issuance, title, author, status, organizational division, branch, section, and any historical information regarding past versions. The QA Manager (or designee) is generally the individual responsible for maintaining a file listing all current quality-related SOPs used within the organization. If an electronic database is used, automatic “Review SOP” notices can be sent. Note that this list may be used also when audits are being considered or when questions are raised as to practices being followed within the organization.